Understanding Supplemented Foods: A Guide to Interpreting Product Labels
Have you noticed that certain food products on store shelves look different from conventional foods? You might be wondering what they are and how you should approach them.
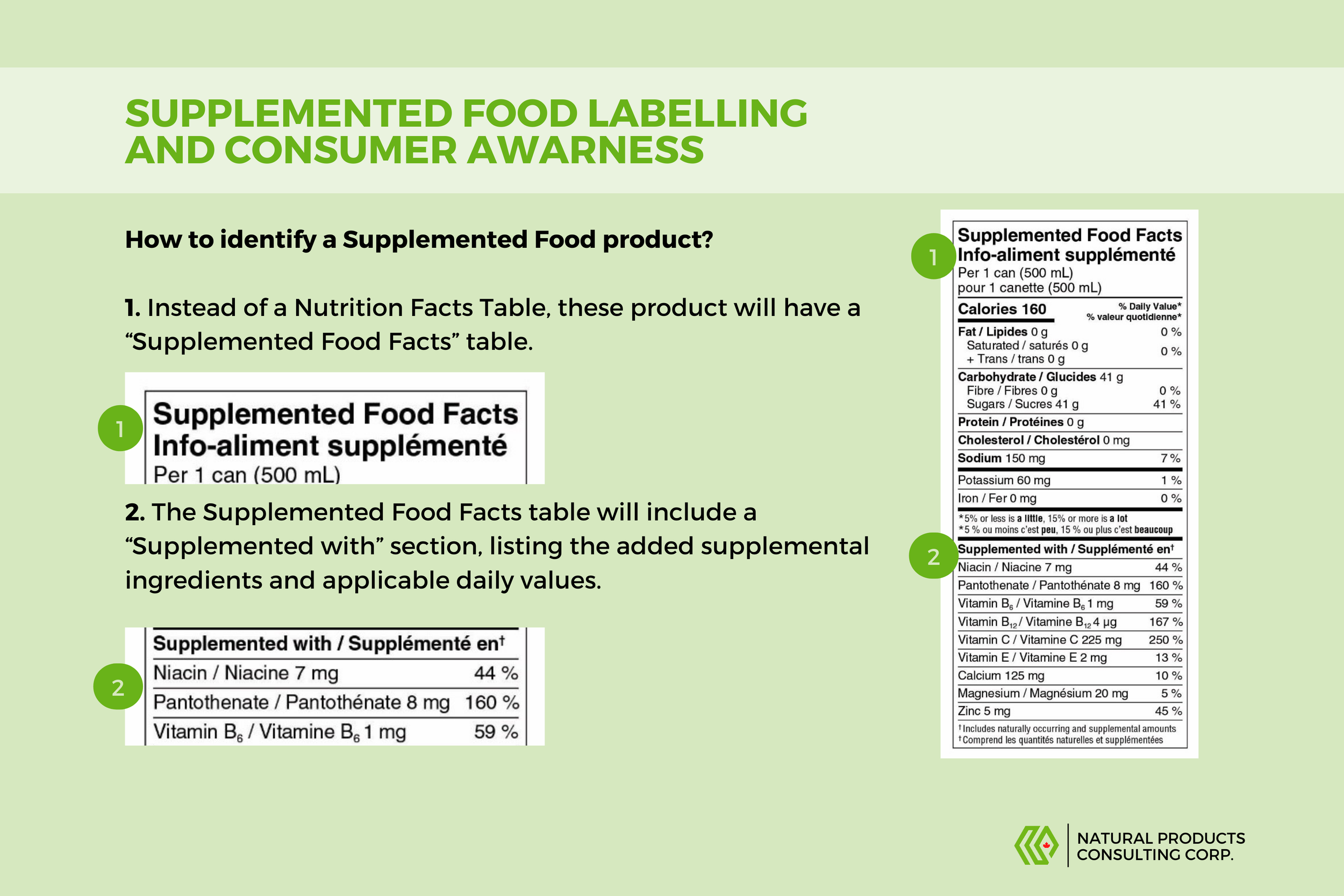
Our team has created a helpful graphic to introduce you to Supplemented Foods, a new food category that has been added to Canadian regulations. These foods contain additives such as vitamins, amino acids, minerals, and caffeine. Due to their unique nature, additional precautions are taken to ensure that consumers are aware of the supplemented ingredients added to such products, preventing potential overconsumption and resulting adverse effects.
It's safe to use these products according to the conditions listed on the product, and consumers are encouraged to familiarize themselves with the caution statements and directions of use that may appear on these products prior to use.
Check out our graphic below to learn more about product labels and how to interpret them while shopping. If you're a manufacturer that's looking to learn more about the applicable regulations, contact our team at info@npc-corp.ca, and we'll be happy to help.
Manufacturers of supplemented foods have until December 31st, 2025 to be fully compliant with applicable regulations.
Follow us on LinkedIn